 By Mohan December 5, 2025
min read
By Mohan December 5, 2025
min readWhy Healthcare AI Compliance Is Non-Negotiable
Everyone is excited about AI right now.
Every boardroom discussion, every product roadmap, every investor pitch – all pointing to the same belief: AI will redefine how industries operate.
But here’s the part most industries don’t have to worry about: a single AI decision in healthcare can affect someone’s life, not just a KPI.
An incorrect prediction, a biased model output, a misaligned dataset – and suddenly you’re not dealing with a “tech issue,” you’re dealing with clinical risk, legal exposure, and regulatory scrutiny.
That’s why healthcare can’t adopt AI the way e-commerce or fintech does. With FDA oversight in the United States, emerging global frameworks, and penalties that can reach millions of dollars, healthcare organizations must navigate complex compliance landscapes before deploying AI solutions.
Here, innovation and compliance must move together – or not at all.
So, before you deploy that diagnostic model, automate a workflow, integrate an AI chatbot, or plug AI into your EHR, you must understand one thing:
AI in healthcare succeeds only when it is compliant with laws, with ethics, and with patient safety.
This blog breaks down the essential compliances you MUST follow while leveraging AI in healthcare – so your organization can innovate confidently, mitigate risk, and build trust from day one.
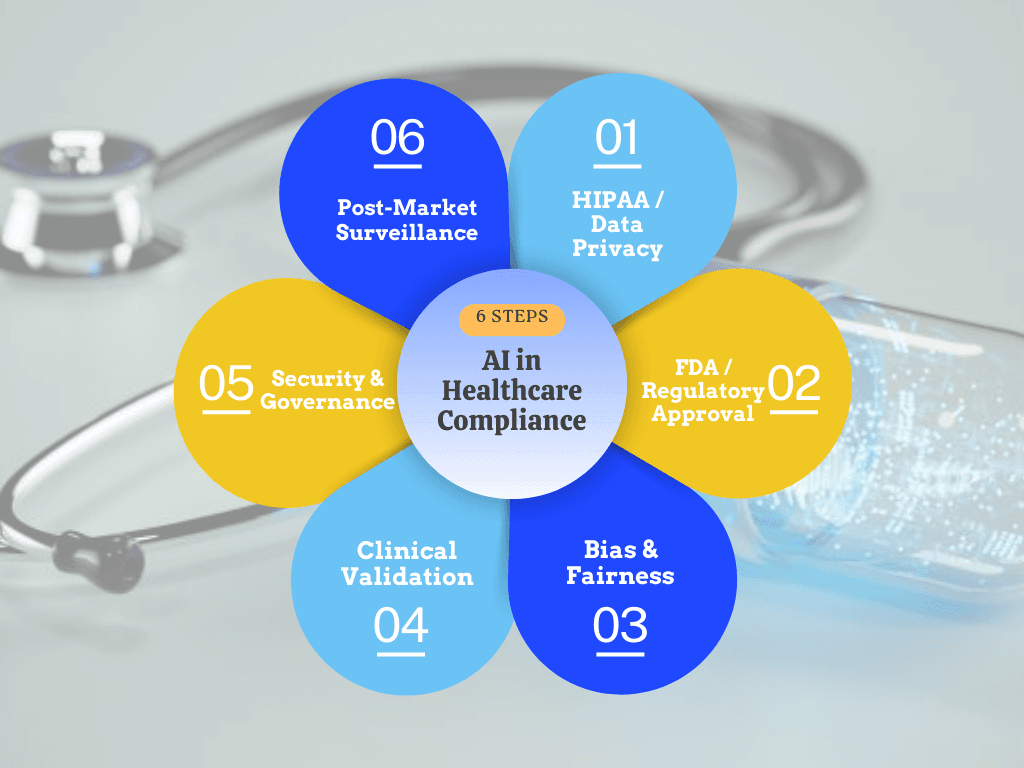
The Real Cost of Non-Compliance
Healthcare AI impacts patient safety directly, making it subject to medical device regulations, data protection laws, and ethical standards simultaneously.
Non-compliance consequences:
- Product recalls and market withdrawal
- Legal liability for patient harm
- Multi-million-dollar penalties (up to €35 million/$41 million globally under EU regulations) [1]
- Loss of business partnerships and procurement eligibility
- Irreparable reputational damage
FDA Requirements: US Regulatory Foundation
The FDA regulates AI as Software as a Medical Device (SaMD) when used for diagnosis, treatment, or disease prevention. With over 950 approved AI/ML medical devices, regulatory pathways are well-established.
Key FDA Compliance Requirements:
Predetermined Change Control Plans (PCCPs)
Finalized in 2024, PCCPs allow pre-approved AI model updates without repeating full authorization, including modification descriptions, validation protocols, and impact assessments.
Quality System Regulation (QSR)
Mandatory documentation of design processes, training data specifications, testing methodologies, risk analysis, and version control.
Post-Market Surveillance:
- Continuous performance monitoring
- Adverse event detection and reporting
- Real-world data collection
- Corrective action implementation
FDA Compliance Steps:
- Classify the AI system risk level (Class I, II, or III)
- Prepare technical and clinical documentation
- Submit premarket application (510(k), De Novo, or PMA)
- Establish ISO 13485-aligned quality management
- Implement post-market monitoring protocols
HIPAA: Protecting Patient Data
HIPAA governs how AI systems handle Protected Health Information (PHI).
Critical HIPAA Requirements:
Business Associate Agreements (BAAs)
Required for all AI vendors processing PHI, specifying access restrictions, security safeguards, breach notifications, and audit rights.
Privacy Rule Compliance:
- Minimum necessary PHI use
- Patient consent documentation
- Individual access rights
Security Rule Implementation:
- Administrative: Risk assessments, workforce training, contingency planning
- Physical: Facility access controls, workstation security
- Technical: Encryption (data at rest/transit), access controls, audit logging
Breach Notification
Notify affected individuals and HHS within 60 days of breaches affecting 500+ people.
HIPAA Safe Harbor Protection
Organizations implementing proper encryption for PHI at rest and in transit qualify for Safe Harbor protection. If encrypted data is breached, organizations are exempt from breach notification requirements and associated penalties, provided encryption meets NIST standards (AES 256-bit or equivalent).
Critical Warning
Never input PHI into public AI tools like ChatGPT, this violates HIPAA. Organizations must establish policies preventing unauthorized PHI processing and monitor AI tool usage.
Global Compliance Overview
European Union: The EU AI Act (effective August 2025) classifies healthcare AI as high-risk, requiring technical documentation, risk management (ISO 14971), representative datasets, human oversight, and transparency. Penalties reach 7% of global turnover [1].
Other Markets:
- UK: MHRA, ICO, and CQC oversight
- India/Middle East: Emerging frameworks emphasizing transparency and safety
Global Convergence: Despite jurisdictional differences, universal themes include transparency, lifecycle management, data privacy, and mandatory human oversight.
Critical Compliance Areas
Addressing Bias and Health Equity
The FDA prioritizes health equity, defining bias as systematic treatment differences across populations.
Bias Mitigation:
- Test across diverse demographic groups
- Use representative training datasets
- Conduct independent validation audits
- Monitor performance disparities continuously
- Implement corrective updates when bias is detected
Bias Types:
- Data: Underrepresentation in training data
- Algorithmic: Model design favors the majority
- Deployment: Unequal care access
Transparency and Explainability
AI cannot function as a “black box” in healthcare.
XAI Requirements
- Document decision-making logic
- Provide clinician-friendly explanations
- Enable patient-facing information
- Maintain audit trails
- Allow human override
Mandatory Human Oversight
Healthcare providers must make final clinical decisions, with AI supporting but never replacing judgment.
Oversight Standards:
- Clinicians can override recommendations
- Continuous qualified personnel monitoring
- Escalation procedures for anomalies
Cybersecurity Standards
Essential Measures:
- End-to-end encryption
- Multi-factor authentication
- Regular penetration testing
- Incident response plans
- ISO/IEC 42001:2023 and ISO 27001 compliance
Implementation Best Practices
Compliance-by-Design
Build from Inception:
- Integrate regulatory requirements at project start
- Establish data governance frameworks
- Implement privacy-by-design principles
- Create AI-specific organizational policies
- Develop ISO 13485-aligned quality systems
Vendor Evaluation Checklist
- FDA clearance or regulatory approval
- HIPAA compliance and valid BAA
- SOC 2 Type II and ISO 27001 certifications
- Clinical validation with peer-reviewed studies
- Post-market surveillance capabilities
- Transparent algorithm documentation
- Demonstrated bias mitigation
- Security audit reports
Essential Documentation
Maintain comprehensive records of algorithm specifications, training data provenance, testing methodologies, risk analysis, version control, clinical validation, regulatory submissions, data processing agreements, and adverse events.
Training Requirements
- Clinical Staff: Understand AI limitations, interpret outputs, and maintain judgment.
- Compliance Officers: Know regulatory frameworks, risk assessment, and audit procedures.
- Technical Teams: Secure development, bias detection, privacy-preserving methods
Post-Market Surveillance
Continuous Monitoring:
- Clinical outcome validation
- Demographic performance tracking
- Model drift detection
- User feedback analysis
Update Management:
- Evaluate submission requirements
- Implement PCCP-approved changes
- Communicate updates with labeling
- Validate effectiveness
- Document modifications
Competitive Advantage Through Compliance
Market Benefits:
- Faster regulatory approvals
- Enhanced stakeholder trust
- Preferred vendor status
- Reduced liability
- Premium pricing justification
Operational Benefits:
- Streamlined development
- Better risk management
- Efficient audits
- Stronger culture
Action Plan for Decision-Makers
- Conduct regulatory assessment
- Establish a cross-functional governance team
- Implement data governance frameworks
- Develop AI-specific policies
- Build documentation systems
- Create vendor evaluation protocols
- Deploy continuous monitoring
- Invest in AI literacy training
- Engage regulatory consultants
- Plan for an international scale
Why Partner with Fortunesoft for Compliant AI Healthcare Solutions?
Because you need a partner who treats compliance as a core engineering principle, not a checkbox.
Our Compliance Strengths:
- 16+ years building healthcare software for payers, providers & healthtech enterprises
- FDA (SaMD) compliant development workflows with PCCP-ready documentation
- HIPAA-aligned engineering + mandatory BAAs for all PHI-driven projects
- GDPR & global privacy compliance for multinational deployments
- ISO 13485 and ISO 27001 – guided quality processes that strengthen safety, reliability, and secure product delivery.
- Privacy-by-design & security-by-design embedded from day one
- Clinical-grade validation with demographic performance testing to minimize bias
Fortunesoft combines deep compliance expertise, AI engineering maturity, and domain-driven architecture to help you build trustworthy, scalable, and regulation-aligned Healthcare Technology Solutions.
We don’t just ship features; we ensure every workflow, integration, and data flow stands up to regulatory scrutiny while accelerating your go-to-market.
Conclusion
Healthcare AI compliance is the foundation for sustainable, trustworthy deployment, protecting patients while delivering value. Ensuring AI compliance in healthcare demands proactive leadership, cross-department collaboration, and a culture of continuous improvement.
Core principles remain constant: patient safety first, data protection always, transparency throughout, human oversight mandatory.
Organizations embracing regulatory requirements as a competitive advantage will lead the healthcare AI revolution. Start building your compliance-first strategy today.
FAQs
1. What regulations govern AI in healthcare in the United States?
AI in healthcare is regulated by the FDA (for medical devices) and HIPAA (for patient data). The FDA classifies clinical AI as Software as a Medical Device (SaMD) requiring premarket clearance. HIPAA mandates strict security controls for any AI processing of Protected Health Information, including Business Associate Agreements with vendors.
2. Does my AI tool need FDA approval?
Yes, if your AI diagnoses conditions, recommends treatments, predicts disease progression, or analyzes medical images for clinical decisions.
No, if your AI only handles administrative tasks (scheduling, billing) or provides general wellness information without medical claims.
Most clinical AI systems require 510(k) clearance as Class II devices.
3. What are Business Associate Agreements (BAAs)?
A BAA is a legally required contract between healthcare organizations and vendors processing Protected Health Information. It specifies security requirements, breach obligations, and audit rights. Without a valid BAA, using AI vendors creates HIPAA violations with penalties up to $1.5 million annually per category.
4. What is HIPAA Safe Harbor?
Safe Harbor exempts organizations from breach notification requirements when data was properly encrypted (AES 256-bit NIST standards). If encrypted PHI is breached, you avoid notification requirements and associated penalties, making encryption a critical compliance investment.
5. How do I ensure my AI complies with bias requirements?
- Test across diverse demographic groups (race, ethnicity, age, gender)
- Use representative training data reflecting real-world populations
- Monitor performance disparities continuously post-deployment
- Conduct independent validation audits
- Document all mitigation efforts for regulatory submissions
6. What are Predetermined Change Control Plans (PCCPs)?
PCCPs allow pre-approved AI model updates without repeating full FDA submissions. They include modification descriptions, validation protocols, and monitoring methods—enabling continuous improvement while maintaining compliance and reducing regulatory costs.
7. Can I use ChatGPT or public AI tools for healthcare?
Absolutely NOT with patient data. Entering PHI into public AI tools violates HIPAA. These platforms lack Business Associate Agreements and cannot protect patient information. Use only HIPAA-compliant, BAA-covered AI solutions for clinical work.
8. What documentation is required for AI compliance?
- Algorithm specifications and training data provenance
- Testing methodologies with demographic performance metrics
- Risk analysis and mitigation strategies
- Clinical validation studies and regulatory submissions
- Business Associate Agreements and adverse event reports
- Quality management records and version control logs
Maintain all documentation for device lifecycle plus 7 years minimum.
9. What are the penalties for non-compliance?
US: HIPAA fines up to $1.5 million per category annually; FDA enforcement includes product seizures and criminal prosecution.
Global: EU AI Act fines up to €35 million ($41 million) or 7% of global revenue; GDPR fines up to €20 million or 4% of revenue.
Beyond financial penalties: market withdrawal, liability for patient harm, and reputational damage.
10. How long does FDA approval take, and what does it cost?
Timeline:
- 510(k) clearance: 3-12 months
- De Novo: 6-12 months
- PMA: 12-18+ months
Includes FDA fees, clinical studies, consulting, and quality system setup.
Sources
- Article 99: Penalties
- FDA: AI/ML in Software as a Medical Device
- HIPAA Security Rule: 45 CFR Part 164
- ISO 13485: Medical Device QMS
- ISO/IEC 42001: AI Management Systems
Author Bio


 Facebook
Facebook Whatsapp
Whatsapp LinkedIn
LinkedIn Pinterest
Pinterest














 Start Chat
Start Chat